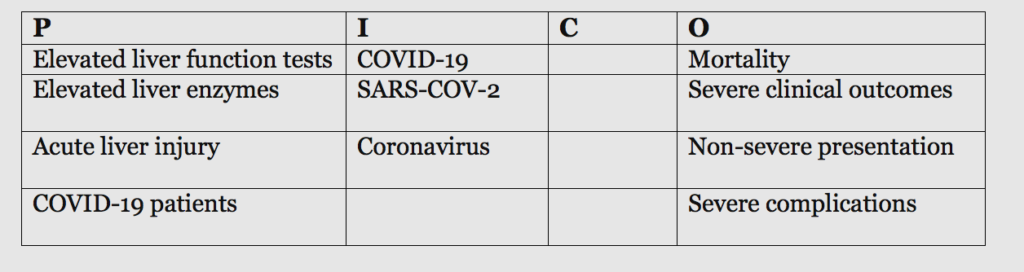
How is abnormal liver function test correlated with the severity and prognosis of COVID-19 positive patients?
Scenario: 7 y/o Hispanic female presented to pediatric ED of NYPQ with complaints of chest pain, palpitations, SOB and a vomiting episode earlier in the morning. PMHx is significant for asthma and a resolved congenital heart defect. Patient was sent for a chest x-ray, which was WNL. Basic blood work was ordered as well as a COVID-19 swab, which came back positive. LFTs were significant for elevated ALP (500), ALT (230) and AST (155). Patient was given supportive treatment and discharge with specific instruction to do quarantine. This patient’s presentation was uneventful to me, except for the LFTs results. As per the attending, this abnormality has been observed in COVID-19 patients. Therefore, I would like to investigate the correlation between abnormal liver function tests and COVID-19 patients.
Purpose: To determine the correlation between abnormal liver function tests and COVID-19 infections as well as infection outcomes related to liver tests abnormalities.
Appraised: Daniela Arias Rodriguez
Date of completion: 10/4/2020
Question: How is abnormal liver function test correlated with the severity and prognosis of COVID-19 positive patients?
PICO search terms
PICO Search Strategy: For this search, I used the terms “COVID-19 and liver injury” in three different databases. I was very successful at finding systematic review and meta-analysis addressing this topic in Pubmed. Most of the articles studying liver injury related to COVID-19 were accessible in this database and, of course, were published within this year. I was not very successful in the Cochrane library, so I decided to do the entire search in Pubmed.
Pubmed:
COVID-19 and Liver Injury→ 229 results
COVID-19 and Liver injury–> (best match, 10 years, RCT, meta analysis, systematic review, clinical trial, full text) –>21 results
Google Scholar:
COVID-19 and Liver Injury–> 67,700 –> (filter 2010-2020) → 16,600
Cochrane Library:
COVID-19 and liver injury –> (filter 2010-2020) –> 13 trials and 0 Cochrane review
Chosen Articles:
1. Kumar-M, P., Mishra, S., Jha, D. K., Shukla, J., Choudhury, A., Mohindra, R., . . . Sharma, V. (2020). Coronavirus Disease (COVID-19) and the Liver: A comprehensive systematic review and meta-analysis. doi:10.21203/rs.3.rs-37723/v1
- 2. Kulkarni, Anand V., et al. “Systematic Review with Meta-Analysis: Liver Manifestations and Outcomes in COVID-19.” Alimentary Pharmacology & Therapeutics, vol. 52, no. 4, 2020, pp. 584–599., doi:10.1111/apt.15916.
3. Zarifian, Ahmadreza, et al. “Gastrointestinal and Hepatic Abnormalities in Patients with Confirmed COVID‐19: A Systematic Review and Meta‐Analysis.” Journal of Medical Virology, 2020, doi:10.1002/jmv.26314.
4. Wu, Yanyan, et al. “Incidence, Risk Factors, and Prognosis of Abnormal Liver Biochemical Tests in COVID-19 Patients: a Systematic Review and Meta-Analysis.” Hepatology International, 2020, doi:10.1007/s12072-020-10074-6.
5. Youssef, Mohanad, et al. “COVID‐19 and Liver Dysfunction: A Systematic Review and Meta‐Analysis of Retrospective Studies.” Wiley Online Library, John Wiley & Sons, Ltd, 27 July 2020, onlinelibrary.wiley.com/doi/abs/10.1002/jmv.26055.
Evidence Retrieved
Kumar et al, 2020
Level of evidence: Systematic-review and meta-analysis
Sample/settings:
- sample size: This analysis included a total of 128 studies. The study does not specifically state the sample size.
- Inclusion criteria: studies were included if they reported frequency of liver abnormalities (abnormal serum bilirubin, ALT, AST, ALP, GGT) in patients infected with COVID-19. Studies were also included if they reported the median and mean levels of severe and non-severe disease or reported baseline liver disease as comorbidity in infected patients.
- Exclusion criteria: Studies with sample size less than 5 patients were excluded as well as studies with unconfirmed COVID-19 cases or with unreported liver functions or baseline liver diseases.
- Primary outcomes: pooled prevalence of abnormal liver function test in COVID-19 cases and severe/non-severe cases, liver abnormalities in COVID-19/non-COVID patients, pooled prevalence of underlying liver disease in COVID-19 patients and severe/non-severe.
Key findings:
- The pooled prevalence of hyperbilirubinemia was 10.98%, while for hypoalbuminemia was 61.27%. The pooled prevalence of elevated alanine aminotransferase (ALT) was 23.28% and for aspartate aminotransferase 23.41%. The pooled prevalence of elevated ALP and GGT were 7.48% and 27.94%, respectively.
- The pooled frequency of hyperbilirubinemia in severe COVID disease was 18.80%, while in non-severe COVID cases it was 9.24%.
- The risk ratio of abnormal bilirubin in severe as compared to non-severe cases was 1.82% with standard mean difference of bilirubin concentration of 0.43.
- The pooled frequency of elevated ALT in severe cases was 39.58% and 24.15% in non-severe COVID cases. The pooled frequency of elevated AST was 49.68% in severe cases and 19.40% in non-severe COVID cases.
- The pooled prevalence of elevated ALP in severe cases was 11.33% and 4% in non-severe, while for GGT it was 46.90% and 18.66% for severe and non-severe cases, respectively.
- In terms of pooled prevalence of albumin abnormalities, it was 75.91% and 31.04% in severe and non-severe COVID cases, respectively.
- The pooled prevalence of chronic liver disease in severe disease was 3.03% and 2.20% in non-severe. Frequency of acute hepatic injury was 44.63% and 20.02% in severe and non-severe COVID cases, respectively.
Limitations/ Biases: One of the limitations that is inherited by systematic reviews and meta-analysis is the heterogeneity among the studies. Researchers highlighted the concern for different definitions of liver dysfunction, acute liver injury, normal values of liver enzymes as well as variable definitions of non-severe and severe COVID groups. Analysis of abnormal liver function tests among COVID and non-COVID cases was limited by a small number of patients. Moreover, this study could not determine whether the elevated liver enzymes were caused by multidrug treatment or exacerbation of inflammation as part of the disease process. Lastly, studies included in this systematic review and meta-analysis were performed in China and therefore, might not accurately represent the US population.
Kulkarni et al, 2020
Level of evidence: systematic review and Meta-analysis
Sample/Settings:
- Sample size : It included 107 studies with a total sample size of 20,874 patients. Of the 107 studies, 92 were conducted on adults, 11 on the pediatric population, 4 on adults and the pediatric population. All the participants were infected with COVID-19.
- Inclusion criteria: Case reports, case series, cross-sectional studies, case-control, retrospective/prospective cohort, quasi-randomized and randomized control trials reporting elevated liver tests in patients with COVID-19 met criteria for inclusion.
- Exclusion criteria: studies not reporting liver tests or preexisting chronic liver disease were subjected to exclusion. Review articles, virology studies, studies addressing mechanism, epidemiology, transmission, use of artificial intelligence, recommendations, and surveillance were also excluded.
- Outcomes: number and outcomes of patients with COVID-19 and liver diseases, pooled incidence of elevated liver tests at presentation and during COVID infection, incidences of AST/ALT, hyperbilirubinemia/hypoalbuminemia, ALP/GGT elevation at initial presentation of COVID-19 infection as well as incidence of prolonged PT and drug-induced liver injury in infected patients.
- Underlying chronic liver disease was defined as preexisting liver disease before being infected with SARS-CoV-2 like cirrhosis, chronic hepatitis C and B, non-alcoholic fatty liver disease (NAFLD) and autoimmune hepatitis.
- Severe liver injury was defined as elevation of liver enzymes above 3 times the upper limit of normal and bilirubin elevation above 2 times the upper limit of normal.
- Severe infection was defined as respiratory rate>30 bpm, mean oxygen saturation of <93%, FiO2 <300mmhg, CT showing lesion progression of 50% within 24-48hrs as well as severe pneumonia. Patients meeting one major criteria or 3> minor criteria of the American Thoracic Society guidelines for community-acquired pneumonia were classified as severe pneumonia.
Key Findings:
- Fifty articles reported underlying liver disease of which 409 patients had a preexisting liver condition. Among the 15,407 infected patients, the pooled prevalence of chronic liver disease was 3.6%.
- The incidence of elevated liver chemistry in COVID-19 patients varies from 1.1% to 68%. The pooled incidence of elevated liver chemistry test was 23.1% in a total of 13,056 infected patients. Among 12,756 infected adults, the incidence of elevated liver tests was 24.1% and in children 17.8% from a total of 283 patients. In non-survivors, the incidence of elevated liver chemistries was 43.3% as compared to survivors with an incidence of 19.2%.
- At presentation, non-survivors had higher risk of having elevated liver chemistry tests as compared to survivors (OR:-3.46, p<0.001).
- Among 12, 778 patients, the pooled incidence of mortality was 12.7%. Three studies from the US reported a mortality of 38.5%. The pooled incidence of mortality in children was 2.3%.
- The incidence of elevated liver chemistries in patients with non-severe infection was 19.9% from a total of 1290 patients and 41.1% for severely infected patients from a total of 780 ill patients.
- Eighteen articles reported elevated liver chemistries during the infection. From a total of 5762 patients, 24.4% had elevated liver chemistries.
- From 3440 patients, the incidence of severe liver injury was 10.7%. This incidence was 24.9% for non-severe patients (n=358) and 41.5% for severely infected patients (n=317).
- The pooled incidence of AST elevation was 22.5% among 11,914 adults patients, while incidence of ALT elevation was 20.1% among 11,431 adults.
- The incidence of hyperbilirubinemia was 13.4 % among 3248 adult patients, while the incidence of ALP elevation was 6.1%. Incidence of GGT elevation was 21.1% among 972 patients, while for hypoalbuminemia was 55.5% among 1990 patients. In severe patients, hypoalbuminemia was reported in 72.9% of the patients and in non-severe it was 1.1-45.8%.
- The pooled incidence of drug-induced liver injury was 25.4%. From a sample of 208 patients treated with remdesivir, drug-induced liver injury was 15.2%. From a sample of 775 patients treated with lopinavir/ritonavir, incidence of drug-induced liver injury was 37.2%.
Limitations / Biases: A limitation of this study was the heterogeneity among the studies and inconsistent classification of liver disease. Moreover, 95 out 107 studies were conducted on China which might not be representative of the US population. Only 7627 out of 20,874 patients were non-chinese patients.
Zarifian et al, 2020
Level of evidence: systematic review and Meta-analysis
Sample/Settings:
- Sample size: it included 67 studies with a total sample size of 13,251 patients infected with COVID-19.
- Inclusion criteria: studies reporting liver biomarkers and GI symptoms in patients with confirmed COVID-19 infection by PCR met criteria for inclusion.
- Exclusion criteria: Comments, letters, review articles, communication, questionable COVID-19 diagnosis were excluded.
- Outcomes: included GI symptoms such as diarrhea, vomiting, nausea, anorexia, abdominal pain/distention and GI comorbidities. Other outcomes included indicators of liver function (ALT, AST, ALP, albumin, total bilirubin and prothrombin time).
Key Findings: - The most common liver function test abnormalities included mild decreased albumin (39.8%), mild increased AST (22.8%) and ALT (20.6%)
- Other liver function test abnormalities were elevated total bilirubin (7.8%), elevated prothrombin time (18%) and ALP (4.6%).
- The most common identified GI symptoms included anorexia (10.2%), diarrhea (8.4%), and nausea (5.7%).
- Chronic liver and GI diseases were present in 5.9% of patients with COVID-19.
- Anorexia and decreased albumin were the most common GI and hepatic abnormality found in patients with COVID.
- As compared to patients with non-severe infection, anorexia was two times more prevalent in patients with severe infection as well as diarrhea, vomiting and abdominal pain.
- Patients with severe infection had higher prevalence of abnormal liver function and underlying diseases.
Limitations / Biases: One limitation of this study is the heterogeneity across the study and the diverse criteria for severity of COVID infection. Because China was the center of the initial epidemic, most available studies were conducted in this country. Therefore, there are not enough studies with large population size performed outside of China.
Wu Y et al, 2020
Level of evidence: Systematic Review and Meta-Analysis
Sample/Settings:
- Sample size: This study included a total of 45 articles of which 44 were conducted in China and one study in Singapore. The total sample size was not explicitly stated.
- Inclusion criteria: Studies that reported incidence and risk factors of abnormal liver tests were eligible for inclusion.
- Exclusion criteria: Duplicates, comments, case reports, reviews, meta-analyses, guidelines, consensus, experiment/animal studies, notes, letters and unrelated papers, studies with undetailed data and duplicate study population were excluded from the analysis.
Key Findings: - On admission, the pooled incidence of abnormal liver biochemical tests was 27.2%.
- During hospitalization, the pooled incidences of abnormal ALT, AST and TBIL (total bilirubin) were 38.4%, 28.1% and 23.2%, respectively.
- At admission, the pooled incidence of abnormal ALT, AST, ALP, GGT, TBIL and ALB were 20.4%, 21.8%, 4.7%, 35.8%, 8.8% and 39.8%, respectively.
- The meta-analyses found that patients with severe COVID-19 disease had higher incidence of abnormal AST upon admission. Incidence of abnormal ALT and TBIL was not significant between patients with severe and non-severe disease upon admission.
- When compared to patients with moderate and mild disease, critical patients and patients with severe disease had higher incidence of any abnormal liver biochemical, AST, ALT, TBIL test at admission.
- ALT levels were found to be higher during hospitalization as compared to admission. ALT levels were higher during the second week of hospitalization as compared to the first week.
- Meta-analyses showed that non-survivors had greater incidence of abnormal liver tests (RR:1.34, P=0.04)
Limitations / Biases: One of the limitations of this study was the heterogeneity across the studies as well as the different definition of severe COVID disease. For example, 36 studies used the Chinese guidelines to diagnose/treat COVID and classify patients under mild, moderate, severe and critical infection. Five studies used the American Thoracic Society guidelines to categorize patients as severe and non-severe infection. As occurred with the previous systematic-reviews and meta-analysis, most of the studies were performed in China (44 out of the 45 studies) which means that results might not be as applicable to the US population. Most of the studies were retrospective cohorts which could have introduced recall bias during data collection.
Youssef M et al, 2020
Level of evidence: Systematic Review and Meta-analysis of Retrospective Studies
Sample/setting
- Sample size: It included 20 retrospective cohort studies with a total sample size of 3428 COVID-19 positive patients of which 57.8% were males. Included studies were published between January 30 and April 16, 2020.
- Inclusion criteria: observational, retrospective/prospective cohort, case-control, clinical trials reporting liver function test (ALT, AST, PT, bilirubin, and albumin) of COVID-19 patients were subjected to inclusion. Studies including patients with COVID-19 diagnosis. Studies classifying patients into mild cases of COVID-19 (patients not requiring extensive management) and severe cases (patients developed ARDS or respiratory failure)
- Exclusion criteria: duplication data, case report, case series, only abstract, conference articles, comments, editorials, expert opinions, or studies were not enough data were subjected to exclusion.
Key Findings: - Patients with severe presentation of COVID were found to have high levels of AST (P<0.001), ALT (P<0.001), bilirubin (p<0.001), and PT (p<0.001) as well as lower albumin levels (p<0.001)
- Patients with hypertension (OR: 2.37), chronic kidney disease (OR: 7.28) and diabetes (OR: 2.72) had twice the risk of developing a severe form of COVID, while patients with cardiovascular (OR: 5.11) or cerebrovascular diseases (5.73) had five times the risk of developing severe features.
- Cancer patients also developed severe presentations of COVID-19 (OR: 2.20).
- This study found that patients with severe COVID-19 had a higher chance of developing acute respiratory syndrome (OR: 18.84, p<0.0001) and sepsis (OR: 21.19, P<0.001). In addition, acute liver injury (OR: 1.93, P= 0.001) and acute kidney injury (OR: 7.2, P<0.001) were more observed in patients with severe COVID disease.
Limitations/ Biases: Because this systematic-review and meta-analyses included retrospective cohort studies, it was subjected to recall or selection biases. Moreover, researchers acknowledge that it was difficult to determine if the liver dysfunction observed in COVID-19 patients was the actual results of acute liver injury or worsening of chronic liver disease. Since most of the studies were conducted in China, studies results might not be as applicable to other racial groups; thirteen studies were from Wuhan City, three from Zhejiang, one from Guangdong, one from Hubei, and one from Anhui.
Conclusion(s)
Kumar et al, 2020 This study found that the most common liver function abnormality observed in COVID-19 positive patients was hypoalbuminemia. Elevated alkaline phosphatase, bilirubin, aminotransferase and gamma-glutamyl transferase were also observed in these patients. When severe and non-severe cases were compared, abnormal hypoalbuminemia, GGT, aminotransferase and bilirubin were more common in severe COVID-19 cases. Elevated AST was more pronounced than ALT in severe cases as well as GGT.
Kulkarni et al, 2020 This study found that abnormal liver chemistry is common during COVID-19 infection and at presentation; its severity can correlate with COVID-19 outcomes. In patients with COVID-19, the incidence of elevated liver chemistry can fluctuate from 1.1% to 68%. Moreover, this study showed that hypoalbuminemia is correlated with severe COVID-19 infection. In severe patients, hypoalbuminemia was reported in 72.9% of the patients and in non-severe it was 1.1-45.8%. At the initial presentation, 23.1% of the patients with COVID-19 present with elevated liver chemistries. Moreover, as compared to survivors, non-survivors had higher risk of having elevated liver chemistry tests at presentation (OR:-3.46, p<0.001). The incidence of drug-induced liver injury was 25.4% likely due to remdesivir and lopinavir/ritonavir.
Zarifian et al, 2020 This study showed that the most common GI symptoms and liver manifestation during COVID-19 infection is diarrhea and mild elevation of liver enzymes. However, as compared to patients with non-severe infection, anorexia was two times more prevalent in patients with severe infection. Higher prevalence of abnormal liver function tests were observed in subgroups with severe COVID-19 infection as compared to non-severe subgroups. Common liver function abnormalities include mild decrease in albumin (39.8%) and mildly elevated AST (22.8%) and ALT (20.6%). Common GI symptoms noticed in COVID-19 infected patients include anorexia (10.2%), diarrhea (8.4%), and nausea (5.7%).
Wu Y, 2020 This study found that abnormal liver markers tend to be higher during hospitalization than at admission suggesting a possible drug toxicity during the hospital stay. This study suggested that the antiviral drug, Lopinavir, which is given to COVID patients could be the culprit of abnormal liver function tests in COVID-19 positive patients. This drug is metabolized by CYP3A4. Common abnormal liver function markers at admission included albumin (39.8%, which was the most frequent), GGT (35.8%), AST (21.8%), TBIL (8.8%) and ALP (4.7%).
Youssef et al, 2020 This study showed that abnormal liver function tests tend to be higher in patients with severe COVID-19 infection. In this study, severe cases of COVID-19 were found to have higher levels of AST, ALT, bilirubin and lower albumin levels. Postmortem liver biopsy indicated liver injuries likely caused by the virus itself or drugs used for COVID-19 treatment. This study concluded that patients presenting with abnormal liver function tests are at greater risk of having more severe outcomes.
The studies’ results agreed that abnormal liver function tests are correlated with severe presentation of COVID-19 infection. In these patients, the most common liver abnormality is hypoalbuminemia as well as elevated AST, ALT, GGT and bilirubin. Although, it is not very clear whether the real cause is the virus itself or the antiviral drugs given to COVID-19 patients such as Lopinavir. According to the literature, COVID-19 utilizes angiotensin-converting enzyme 2 to enter the lungs, kidney and heart cells. Expression of ACE2 is higher in bile ducts cells as compared to the liver cells suggesting that liver injury could possibly be the result of bile duct damage. Regardless of the mechanism of liver injury, special attention must be given to COVID + patients with abnormal liver function tests, since they could be a risk for more severe clinical outcomes.
Clinical Bottom Line:
Weight of the evidence I weighed the articles in the following order Kumar> Kulkarni> Zarifian>Wu>Youssef
Kumar et al All five studies are systematic reviews and meta-analyses and were published in 2020, so I weighed the articles based on population size and number studies included. Therefore, Kumar et al carries the heaviest evidence of my conclusion for having more studies in its analysis. This is a systematic review and meta-analysis of 128 studies addressing abnormal liver function tests in patients infected with COVID-19. It was published in July 2020.
Kulkarni et al is a systematic review and meta-analysis of 107 studies with a total sample size of 20,874 patients. All the participants were infected with COVID-19. This study was published in May 2020. This study directly addresses abnormal liver function tests in infected patients with COVID-19.
Zarifian et al is a systematic review and meta-analysis of 67 studies with a total sample size of 13,251 patients infected with COVID-19. It was published on July 13, 2020. This study addressed abnormal liver chemistry in patients infected with COVID-19 as well as GI symptoms in infected patients.
Wu y et al This is a systematic review and meta-analysis of 45 studies. This study was published on July 9, 2020. The total sample size was not explicitly stated, however, it addressed the question of interest.
Youssef et al I weighed this systematic review and meta-analysis last, since it has the smallest sample size and number of included studies. This study included 20 retrospective cohort studies with a total sample size of 3428 COVID-19 positive patients. It was published on May 9, 2020.
Magnitude of any effects
Kumar et al: The most frequent liver abnormality was hypoalbuminemia with a prevalence of 75.91% in severe and 31.04% in non-severe cases. When comparing severe and non-severe groups, abnormal albumin, elevated GGT, bilirubin, aminotransferase and alkaline phosphatase were more frequent in severe COVID-19 infection.
Kulkarni et al: In non-survivors, the incidence of elevated liver chemistries was 43.3% as compared to survivors with an incidence of 19.2%. At presentation, non-survivors had higher risk of having elevated liver chemistry tests as compared to survivors (OR:-3.46, p<0.001). In severe patients, hypoalbuminemia was reported in 72.9% of the patients and in non-severe it was 1.1-45.8%.
Zarifian et al: The most frequent liver abnormality was decreased albumin with 39.8%, mildly increased AST with 22.8% and ALT with 20.6%. Patients with severe infection had higher prevalence of abnormal liver function and underlying diseases.
Wu Y et al: During hospitalization, the pooled incidences of abnormal liver tests were 38.4% for ALT, 28.1% for AST and 23.2% for TBIL (total bilirubin). At admission, the pooled incidence of abnormal liver tests were 20.4% for ALT, 21.8% for AST, 4.7% for ALP, 35.8% for GGT, 8.8% for TBIL and 39.8% for ALB.
Youssef et al: High levels of AST (p<0.001), ALT (P<0.001), bilirubin (p<0.001) and PT (p<0.001) were more frequent in patients with severe presentation of COVID-19. Patients with severe COVID-19 had a higher chance of developing acute respiratory syndrome (OR: 18.84, p<0.0001) and sepsis (OR: 21.19, P<0.001). Acute liver injury (OR: 1.93, P= 0.001) and acute kidney injury (OR: 7.2, P<0.001) were more observed in patients with severe COVID disease.
Clinical significance (not just statistical significance)
In patients infected with COVID-19, lung manifestations are the most fatal and prominent symptoms and therefore, most research has been devoted to study the lung manifestation of COVID-19. However, SARS-COV-2 can present with gastrointestinal manifestation and liver function abnormalities indicating severe infection and worsening outcomes. One of the most frequent liver abnormalities noticed in severe COVID-19 infection was hypoalbuminemia followed by elevated AST, ALT, GGT, and bilirubin. In terms of GI symptoms, diarrhea was the most common GI manifestation, while anorexia was the most frequent GI symptom in severe COVI-19 cases. These results become relevant in the clinical setting when evaluating patients with GI symptoms, abnormal liver tests and without respiratory complaints indicating COVID. In patients infected with COVID-19 and abnormal liver function tests, particularly hypoalbuminemia, it should raise provider’s concern for a more severe infection of COVID-19 and worsen clinical outcome that could warrant prompt treatment and different patient disposition. Moreover, these results indicate that we should still have a high index of suspicion of COVID-19 infection in patients presenting with GI symptoms such as diarrhea and anorexia.
Any other considerations important in weighing this evidence to guide practiceAs mentioned previously, the majority of the studies included in the systematic reviews and meta-analyses were conducted in China. Therefore, large-scale studies conducted in countries other than China are needed to further understand liver abnormalities and COVID-19 infection in other racial groups. Moreover, more research needs to be done to determine the actual cause of the liver abnormalities noticed in patients infected with COVID-19. There are discrepancies on whether the liver abnormalities are actually caused by the virus itself or the use of multiple antiviral treatments during hospitalization.
JMV-9999-na.pdf
12072_2020_Article_10071.pdf